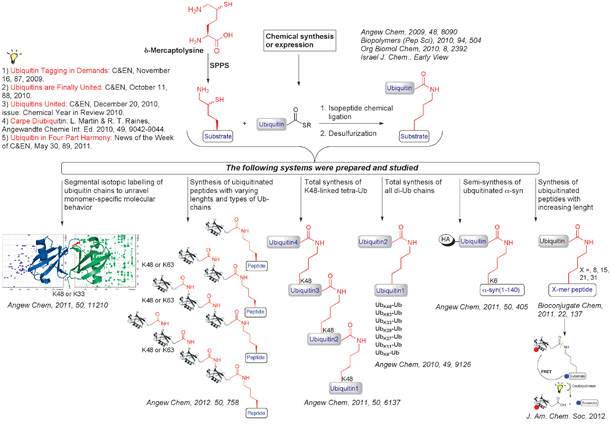
Ubiquitination is the cellular process where a ubiquitin monomer, composed of 76 amino acids, or of a polyubiquitin chain are linked to a protein target, affecting a variety of biological processes such as protein degradation, trafficking, transcription and the DNA damage response. Not surprisingly, ubiquitination plays a key role in various diseases, such as neurological disorders, infectious diseases and cancer. Hence, understanding this signal at the molecular level is crucial for understanding and combating various diseases. In ubiquitination, three enzymes, E1, E2, and E3, collaborate to link the C-terminal Gly of ubiquitin to the Lys side chain of the protein target through an isopeptide bond. Conjugation of the ubiquitin molecule to a protein target may involve an ubiquitin monomer or a chain of ubiquitins of various lengths and linkage types. The evolving complexity of the ubiquitin signal and the recent discoveries of its involvement in a wide range of biological functions continue to dazzle scientists in many ways and hence engage several research groups aiming to decipher the molecular bases of this signal and its importance in health and diseases. Yet, research in this field, including structural and functional analyses, the development of reagents and understanding the enzymatic machineries and the factors involved in this signal, has been challenged by the inability to obtain homogenous ubiquitin bioconjugates at a workable level from cells, and even from cell free reconstituted enzymatic systems where conjugation often proceeds in an uncontrolled manner. We have recently reported a number of novel chemical methods that offer solutions to these challenges and prepare any ubiquitin conjugate in high homogeneity, purity and large quantities to shed light on various processes related to the ubiquitin signal. Specifically, our group has pioneered the development of delta‑mercaptolysine (thiolysine) to mediate the transthioesterification step with ubiquitin thioester, followed by an S-N acyl transfer to form the isopeptide bond between ubiquitin and a specific substrate. The thiol handle can then be removed by applying a desulfurization reaction to furnish the native isopeptide linkage. The above-described tools allowed us, for the first time, to synthesize all Lys linked di-ubiquitin chains in which thiolysine was introduced at the desired position (i.e., K63, K48, K33, K29, K27, K11, or K6), thus facilitating the site-specific attachment of the sequential ubiquitin molecule. The establishment of synthetic/semisynthetic routes for di-ubiquitin chains and analogs by us paved the way for many rigorous studies in order to shed light on the various aspects of ubiquitin systems. It has been shown for example that some small proteins undergo proteasomal degradation with monoubiquitination, however, for other proteins it is known that a chain of four ubiquitin units is necessary to execute the degradation signal. There are numerous cellular activities for which the preferred chain’s length is also unknown. Such questions can be attempted by establishing preparative methods for longer ubiquitin chains anchored to the specific substrate.
Our group was the first to execute the chemical synthesis of K48-linked tetra-ubiquitin chain, which is the largest protein to be assembled chemically to date. In addition, we have also reported the synthesis of peptides linked to mono, di-, tri- and tetra-ubiquitin chains linked via K48 or K63 to examine their behaviors with specific deubiquitinases. our group has also accomplished the semi-synthesis of alpha-syn linked to chains of various lengths and investigated the role of the chain on alpha-synuclein aggregation and phosphorylation by various kinases. More recently, we have also extended these methods for polyubiquitination of expressed proteins and expand the use of these synthetic tools to more complex protein targets.
The recent breakthrough in chemical and semisynthetic approaches reported by our group makes it possible now to assemble ubiquitin chains and ubiquitinated peptides and proteins in high purity and large quantity. As a result, great opportunities have become now available for chemical biologists and biologists to study the ubiquitin signal at the molecular level to reveal unknown aspects of this amazing and diverse signal. Similarly, these tools could be extended to the family of ubiquitin like modifiers, e.g. SOMU, which uses similar enzymatic machinery to link these proteins to their substrates. Along these adventures, chemistry will continue to play a vital role in creating new opportunities in studying these signals and their importance in health and disease.
Recent synthetic targets that were accomplished by our group.