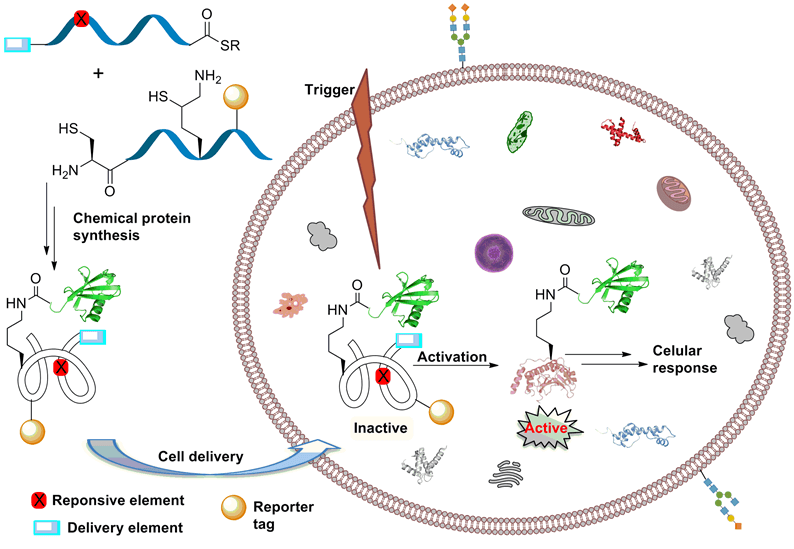
Delivery and On-Demand Activation of Chemically Synthesized and Uniquely Modified Proteins in Living Cells
Despite significant recent advances in protein research, the full scope of many protein cellular functions remains insufficiently characterized. An important strategy used to probe protein function is observing the effect of natural (posttranslational modifications, PTMs) and unnatural modifications on the protein activity. Protein modifications achievable by in vivo molecular biology approaches, however, are severely limited in their ability to introduce PTMs and unnatural modifications, including those that would allow for temporal control of protein function. At the same time, in vitro chemical protein synthesis allows for unlimited protein design and modification, but studies on delivering these synthetic proteins into cells are rare, and are still limited to simple systems lacking atomic complexity, such as PTMs and temporal control elements. In order to answer fundamental questions about the cellular role of many proteins, and to enable monitoring proteins cellular function and fate in real time, the fields of (semi)synthetic protein design and cellular protein manipulation must be bridged by significant advances in methods for protein delivery and real-time activation and monitoring.
In this ERC advanced project we aim to develop a general approach for enabling considerably more detailed in-cell study of uniquely modified proteins by preparing proteins having the following features: 1) traceless cell delivery unit(s), 2) an activation unit for on-demand activation of protein function in the cell, and 3) a fluorescence probe for monitoring the state and the fate of the protein.
We will adopt this approach to shed light on the processes of ubiquitination and deubiquitination, which are critical cellular signals for many biological processes. We will employ our approach to study 1) the effect of inhibition of deubiquitinases in cancer. 2) Examining effect of phosphorylation on proteasomal degradation and on ubiquitin chain elongation. 3) Examining effect of covalent attachment of a known ligase ligand to a target protein on its degradation. Moreover, this could trigger the development of new methods to modify the desired protein in cell by selective chemistries and so rationally promote their degradation.