Posttranslational modifications of proteins play crucial roles in health and disease by affecting numerous aspects of protein structure, function, stability and sub-cellular localization. Yet understanding the effects of these modifications at the molecular level has been hindered by the lack of homogeneously modified proteins obtained via traditional biochemical and molecular biology approaches. Moreover, the preparation of such complex bioconjugates at a workable level is highly demanding. Recent advances in protein chemistry applying chemical and semisynthetic approaches are becoming increasingly beneficial to overcome these challenges. Our lab is interested in developing novel synthetic methods as well as utilizing the available state of art approaches to prepare site-specifically modified proteins for biochemical, biophysical and functional analyses.
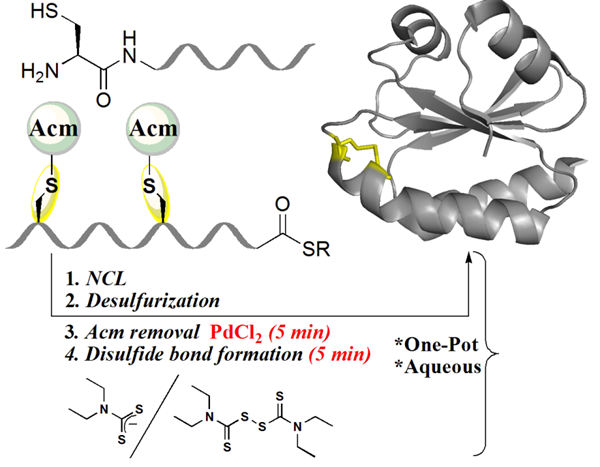
For example, we recently reported that palladium complexes can remove multiple Cys protecting groups within minutes in a fully aqueous medium, which could also be coupled in-situ with native chemical ligation to provide excellent yields of the desired product. We also demonstrated unprecedented palladium chemoselectivity in a fully aqueous medium for on-demand orthogonal deprotection of Thz and Acm. We used this chemistry for one-pot and site-specific modification of peptides containing multiple Cys residues, as well as for the total chemical synthesis of the CSP-1 protein, which contains 13 Cys residues. In addition, we reported the first chemical synthesis of an activity-based probe of ubiquitinated histone H2A at Lys119 for the preparation of ubiquitinated nucleosome core particle probes (NCP-(H2A-UbDHA)). We further extended our palladium chemistry to introduce cleavable linkers which were employed for the attachment of cleavable solubilizing tags to prepare difficult peptides and proteins. This chemistry also further extended for the rapid disulfide bond formation in peptides and proteins containing Cys(Acm). Finally, using this chemistry we developed a strategy for the cellular delivery and on-demand activation of synthetic proteins (see ERC project).